Copper Is Electroplated From Cuso4 Solution. A Constant . A constant current of 3.90 a is applied by an external power supply. A constant current of 4.18 a is applied by an external power supply. Web copper is electroplated from cuso4 solution. How long will it take. Calculate the quantity of electricity (coulombs) necessary to deposit 100.0 g of copper from a cuso 4 solution. Web copper is electroplated from cuso4 solution. Web the concentration of copper ions in the solution is effectively constant. Web copper sulfate (cuso4) provides a source of copper ions. Copper is electroplated from cuso4 solution. A constant current of 10.0 amps is applied by an external power supply for exactly 75.0. Calculate the amount of copper deposited (m) using faraday's law of electrolysis: This is because the electroplating process transfers metal from. A constant current of 4.06 a is applied by an external power supply. M = (q × m) / (n × f) where m is. Sulfuric acid (h2so4) makes the bath conductive and acts as a.
from www.chegg.com
Web copper is electroplated from cuso4 solution. Sulfuric acid (h2so4) makes the bath conductive and acts as a. Web copper is electroplated from cuso4 solution. A constant current of 4.18 a is applied by an external power supply. Web copper is electroplated from cuso4 solution. A constant current of 4.06 a is applied by an external power supply. Web copper sulfate (cuso4) provides a source of copper ions. Calculate the quantity of electricity (coulombs) necessary to deposit 100.0 g of copper from a cuso 4 solution. Calculate the amount of copper deposited (m) using faraday's law of electrolysis: A constant current of 10.0 amps is applied by an external power supply for exactly 75.0.
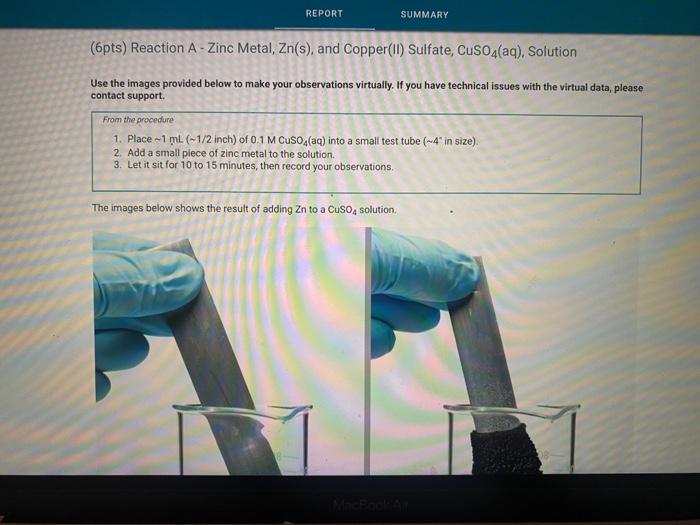
Solved (6pts) Reaction A Zinc Metal, Zn(s), and Copper(II)
Copper Is Electroplated From Cuso4 Solution. A Constant A constant current of 4.06 a is applied by an external power supply. This is because the electroplating process transfers metal from. Sulfuric acid (h2so4) makes the bath conductive and acts as a. A constant current of 4.06 a is applied by an external power supply. Calculate the amount of copper deposited (m) using faraday's law of electrolysis: How long will it take. Web copper is electroplated from cuso4 solution. Web copper is electroplated from cuso4 solution. Calculate the quantity of electricity (coulombs) necessary to deposit 100.0 g of copper from a cuso 4 solution. Web the concentration of copper ions in the solution is effectively constant. A constant current of 3.90 a is applied by an external power supply. Web copper sulfate (cuso4) provides a source of copper ions. Copper is electroplated from cuso4 solution. M = (q × m) / (n × f) where m is. A constant current of 4.18 a is applied by an external power supply. Web copper is electroplated from cuso4 solution.
From www.alamy.com
Copper sulphate solution in a beaker. Copper sulphate (CuSO4) is a salt Copper Is Electroplated From Cuso4 Solution. A Constant Web copper is electroplated from cuso4 solution. A constant current of 10.0 amps is applied by an external power supply for exactly 75.0. Web copper is electroplated from cuso4 solution. M = (q × m) / (n × f) where m is. Web copper is electroplated from cuso4 solution. How long will it take. This is because the electroplating process. Copper Is Electroplated From Cuso4 Solution. A Constant.
From byjus.com
How much copper is deposited on cathode if a current of 3A is passed Copper Is Electroplated From Cuso4 Solution. A Constant A constant current of 3.90 a is applied by an external power supply. How long will it take. M = (q × m) / (n × f) where m is. Web copper is electroplated from cuso4 solution. Web copper is electroplated from cuso4 solution. A constant current of 4.06 a is applied by an external power supply. Copper is electroplated. Copper Is Electroplated From Cuso4 Solution. A Constant.
From www.coursehero.com
[Solved] link for the experiment https//youtu.be/ILJhI43wVA Part Copper Is Electroplated From Cuso4 Solution. A Constant A constant current of 4.06 a is applied by an external power supply. Calculate the amount of copper deposited (m) using faraday's law of electrolysis: How long will it take. Web the concentration of copper ions in the solution is effectively constant. A constant current of 4.18 a is applied by an external power supply. A constant current of 10.0. Copper Is Electroplated From Cuso4 Solution. A Constant.
From www.numerade.com
SOLVED Copper is electroplated from CuSO4. A constant current of 6.00 Copper Is Electroplated From Cuso4 Solution. A Constant A constant current of 4.18 a is applied by an external power supply. Web copper is electroplated from cuso4 solution. Web the concentration of copper ions in the solution is effectively constant. M = (q × m) / (n × f) where m is. Web copper sulfate (cuso4) provides a source of copper ions. Calculate the quantity of electricity (coulombs). Copper Is Electroplated From Cuso4 Solution. A Constant.
From www.numerade.com
SOLVED Copper is electroplated from CuSO4 solution. A constant current Copper Is Electroplated From Cuso4 Solution. A Constant Calculate the quantity of electricity (coulombs) necessary to deposit 100.0 g of copper from a cuso 4 solution. M = (q × m) / (n × f) where m is. Web the concentration of copper ions in the solution is effectively constant. A constant current of 10.0 amps is applied by an external power supply for exactly 75.0. Web copper. Copper Is Electroplated From Cuso4 Solution. A Constant.
From www.numerade.com
SOLVED Copper is electroplated from CuSO4 solution. A constant current Copper Is Electroplated From Cuso4 Solution. A Constant Web copper is electroplated from cuso4 solution. Web copper is electroplated from cuso4 solution. Sulfuric acid (h2so4) makes the bath conductive and acts as a. Calculate the quantity of electricity (coulombs) necessary to deposit 100.0 g of copper from a cuso 4 solution. Web copper sulfate (cuso4) provides a source of copper ions. Web copper is electroplated from cuso4 solution.. Copper Is Electroplated From Cuso4 Solution. A Constant.
From www.numerade.com
VIDEO solution 1 Copper Is Electroplated From Cuso4 Solution. A Constant How long will it take. Calculate the quantity of electricity (coulombs) necessary to deposit 100.0 g of copper from a cuso 4 solution. A constant current of 3.90 a is applied by an external power supply. M = (q × m) / (n × f) where m is. Web copper is electroplated from cuso4 solution. Web copper sulfate (cuso4) provides. Copper Is Electroplated From Cuso4 Solution. A Constant.
From www.youtube.com
Formula mass of CuSO4.5H2O. Copper (II) Sulfate Pentahydrate Copper Is Electroplated From Cuso4 Solution. A Constant A constant current of 4.18 a is applied by an external power supply. Web copper sulfate (cuso4) provides a source of copper ions. Copper is electroplated from cuso4 solution. Calculate the amount of copper deposited (m) using faraday's law of electrolysis: A constant current of 4.06 a is applied by an external power supply. How long will it take. Web. Copper Is Electroplated From Cuso4 Solution. A Constant.
From edu.svet.gob.gt
Cuso4 Colour In Water edu.svet.gob.gt Copper Is Electroplated From Cuso4 Solution. A Constant A constant current of 4.18 a is applied by an external power supply. This is because the electroplating process transfers metal from. Sulfuric acid (h2so4) makes the bath conductive and acts as a. Calculate the quantity of electricity (coulombs) necessary to deposit 100.0 g of copper from a cuso 4 solution. A constant current of 10.0 amps is applied by. Copper Is Electroplated From Cuso4 Solution. A Constant.
From exogbsbxf.blob.core.windows.net
Copper Sulfate Pentahydrate Molar Mass at Peter Franco blog Copper Is Electroplated From Cuso4 Solution. A Constant A constant current of 10.0 amps is applied by an external power supply for exactly 75.0. Copper is electroplated from cuso4 solution. Calculate the amount of copper deposited (m) using faraday's law of electrolysis: Sulfuric acid (h2so4) makes the bath conductive and acts as a. Web copper is electroplated from cuso4 solution. A constant current of 4.18 a is applied. Copper Is Electroplated From Cuso4 Solution. A Constant.
From www.chegg.com
Solved Copper is electroplated from CuS0_4 solution. A Copper Is Electroplated From Cuso4 Solution. A Constant Copper is electroplated from cuso4 solution. A constant current of 3.90 a is applied by an external power supply. A constant current of 4.18 a is applied by an external power supply. A constant current of 4.06 a is applied by an external power supply. This is because the electroplating process transfers metal from. Web copper sulfate (cuso4) provides a. Copper Is Electroplated From Cuso4 Solution. A Constant.
From www.toppr.com
*ELECTRO CHEMISTRY A first order reaction is found to have a rate Copper Is Electroplated From Cuso4 Solution. A Constant M = (q × m) / (n × f) where m is. Copper is electroplated from cuso4 solution. Web the concentration of copper ions in the solution is effectively constant. A constant current of 3.90 a is applied by an external power supply. This is because the electroplating process transfers metal from. Web copper is electroplated from cuso4 solution. A. Copper Is Electroplated From Cuso4 Solution. A Constant.
From www.doubtnut.com
Copper sulphate solution is electrolysed using copper electrodes. Copper Is Electroplated From Cuso4 Solution. A Constant Web copper sulfate (cuso4) provides a source of copper ions. This is because the electroplating process transfers metal from. Web the concentration of copper ions in the solution is effectively constant. How long will it take. M = (q × m) / (n × f) where m is. Sulfuric acid (h2so4) makes the bath conductive and acts as a. Web. Copper Is Electroplated From Cuso4 Solution. A Constant.
From courses.lumenlearning.com
Predicting the Products of Electrolysis Introduction to Chemistry Copper Is Electroplated From Cuso4 Solution. A Constant Web copper is electroplated from cuso4 solution. Copper is electroplated from cuso4 solution. How long will it take. A constant current of 4.18 a is applied by an external power supply. Web copper is electroplated from cuso4 solution. Web copper sulfate (cuso4) provides a source of copper ions. Web the concentration of copper ions in the solution is effectively constant.. Copper Is Electroplated From Cuso4 Solution. A Constant.
From www.alamy.com
Electroplating with copper using copper sulfate electrolyte Copper Is Electroplated From Cuso4 Solution. A Constant A constant current of 3.90 a is applied by an external power supply. This is because the electroplating process transfers metal from. A constant current of 4.06 a is applied by an external power supply. Web copper sulfate (cuso4) provides a source of copper ions. Calculate the amount of copper deposited (m) using faraday's law of electrolysis: Web copper is. Copper Is Electroplated From Cuso4 Solution. A Constant.
From www.coursehero.com
[Solved] . Continuous sheet copper is made by electrodeposition from a Copper Is Electroplated From Cuso4 Solution. A Constant M = (q × m) / (n × f) where m is. Web copper is electroplated from cuso4 solution. Web the concentration of copper ions in the solution is effectively constant. Calculate the quantity of electricity (coulombs) necessary to deposit 100.0 g of copper from a cuso 4 solution. Copper is electroplated from cuso4 solution. Calculate the amount of copper. Copper Is Electroplated From Cuso4 Solution. A Constant.
From www.chegg.com
Solved Copper is electroplated from an aqueous CuSO4 Copper Is Electroplated From Cuso4 Solution. A Constant Web copper sulfate (cuso4) provides a source of copper ions. A constant current of 3.90 a is applied by an external power supply. Copper is electroplated from cuso4 solution. Web copper is electroplated from cuso4 solution. Calculate the amount of copper deposited (m) using faraday's law of electrolysis: This is because the electroplating process transfers metal from. Web the concentration. Copper Is Electroplated From Cuso4 Solution. A Constant.
From www.numerade.com
VIDEO solution Copper is electroplated from CuSO4. A constant current Copper Is Electroplated From Cuso4 Solution. A Constant Web copper is electroplated from cuso4 solution. Web copper sulfate (cuso4) provides a source of copper ions. A constant current of 4.06 a is applied by an external power supply. A constant current of 4.18 a is applied by an external power supply. Web the concentration of copper ions in the solution is effectively constant. How long will it take.. Copper Is Electroplated From Cuso4 Solution. A Constant.